Lipids (mostly known as fats and oil) are organic compounds that include fat and oil. At room temperature, fats and oils are liquids. Lipids contain carbon, hydrogen and oxygen, like carbohydrates, but they have very low oxygen content. Mostly plants have oils which contain unsaturated Oleic acid C18H32O2. Animals on the other hand, store fats containing palmitic acid C16H32O2 or stearic acid C18H36O2 . All lipids are insoluble in water but soluble organic solvents (a typical example being ethanol)
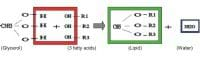
The synthesis of fat is a condensation reaction involving two chemical substances: fatty acid and glycerol.
Three fatty acids combine with glycerol to form a triglyceride, for example tristearin C57H110O6, with the removal of three molecules of water. In digestion, lipids are hydrolysed to fatty acids and glycerol. In mammals fat are stored in adipose tissue under the skin, where they are a food reserve and provide insulation against heat loss. In aquatic mammals, large quantities of synthesis of cell membranes. Weight for weight, fats release about twice (39 kj/mole) the amount of energy that is released by sugar (17.2 KJ/mole) or by protein (approximately 19 kj/mole) when respired.
TESTING FOR LIPIDS
A) Grease spot test
1. Place a spot of oil on a clean sheet of absorbent paper e.g. filter paper
2. Alongside the spot, place a spot of water.
3. leave the sheet of paper for ten minutes, the hold the paper to the light.
Observe the spot that still remains on the paper, is it translucent?
B) Emulsion Test
1. to 2cm3 of ethanol (ethyl alcohol) in a test tube, add a small volume of oil, e.g. groundnut oil,
olive oil, castor oil, palm oil. Shake thoroughly with the thumb over the end of the tube
2. Pour the mixture into a second test tube containing about 2cm3 of water.
Observe what happens on adding the mixture to the water. What does this indicate?
Source:
Please rate this
Poor




 Excellent
Excellent




 Excellent
Excellent
Votes: 1 |4 out of 5